1,2Rochelle Mendonca, PhD, OTR/L, 2Roger O. Smith, Ph.D., OT
1Columbia University, New York, NY, 10032
2Rehabilitation Research Design and Disability (R2D2) Center, University of Wisconsin-Milwaukee
Milwaukee, WI, 53211
ABSTRACT
This study reports the effects of providing medical device accessibility information on purchase decision-makimg by people with disabilities. The study deployed a “discrete choice methodology.” Participants chose one device from four options based on various costs, external opinions, and accessibility scores. Ninety-eight participants with disabilities were included in the results. Participants reported significant preferences for devices with high accessibility compared to devices with low or no accessibility. This study directly links to the a universal design component of the U.S. Affordable Care Act health care legislation, which requires medical devices to be accessible for people with disabilities.
INTRODUCTION
Approximately 40.7 million people with disabilities (12.7% of the US population) live in non-institutionalized settings in the US [1]. Empowering PWD to continue to live at home and be as independent as possible is a fundamental responsibility of modern society. A critical feature of independence is the ability to choose and purchase daily use consumer products, including medical products. PWD represent a large percentage of the population who purchase consumer products, estimated to be valued at about half a trillion dollars [2]. However, there is either limited or no information related to the accessibility of products and services to assist individuals with disabilities make informed choices [3].
A three-level problem statement provides the background for this study. First, medical equipment presents an accessibility barrier to individuals with disabilities [4,5]. Medical equipment includes but is not limited to any furniture, measuring device, device that comes in contact with or is designed to be manipulated, monitored or read by health care professionals, layperson caregivers or end-user patients [6]. Accessibility is defined as “the encounter between a person’s functional capacity and the design and demands of the physical environment” [7,8]. Extensive research shows that people with disabilities encounter difficulties in accessing healthcare, both diagnostic and preventative, in primary care facilities, hospital care and long-term care facilities [9,10]. One of the biggest barriers identified to receiving appropriate and timely healthcare is the inaccessibility of medical equipment [11,12,13].
Second, there is an absence of assessments that can quantify the accessibility of medical devices. Therefore, it is difficult to compare between devices or to provide recommendations for improvements based on scores or specific standard features [14]. Third, there is absence of information to help consumers determine which device will best meet their needs. The provision of accessibility information is sparse in almost all environments and products. In case of medical devices, disuse of devices due to mismatching of needs can be fatal. Moreover, in the U.S. recent legislation mandates that healthcare be accessible to everyone, including people with disabilities. The Patient Protection and Affordable Care Act mandates accessible medical care and specifically speaks to the design of accessible medical devices to be usable by people with disabilities [15]. The United States Access Board developed guidelines for the accessible design of specific diagnostic equipment [16], and the U.S. Department of Veterans Affairs recently adopted medical device accessibility standards to ensure access to medical diagnostic equipment at its health care facilities [17]. Yet despite these requirements, currently there are no methods to measure or quantify the accessibility of medical devices, making it difficult for device designers to compare designs or for healthcare professionals or consumers with disabilities to choose products. This highlights the need for an assessment that can measure the accessibility of medical devices to provide designers, manufacturers and purchasers with valid information to help them design devices that meet the needs of individuals with disabilities. This purpose of this study was to evaluate the usefulness of medical device accessibility information, if provided, on purchase decisions for individuals with disabilities.
METHODS
This study used a post-test only experimental design to investigate the consequences of providing medical device accessibility information on purchase and use for individuals with disabilities. A web-based survey was developed and implemented to evaluate the usefulness of accessibility scores and its relationship to cost and external opinions based on a “Discrete Choice Modeling” (DCM) methodology. Discrete choice models are statistical procedures that describe choices made by people among a finite set of alternatives [18,19]. Participants were shown four different types or models of a medical device. Each of the options had different attributes which were used to determine which attributes and levels of attributes were the most important in participants’ decision-making choices. The attribute information included (a) cost, (b) external opinions and (c) accessibility scores.
The number of choice-sets to be presented and the development of the discrete choice model were determined using Statistical Analysis Software (SAS) macros for multinomial logic. DCM reliably calculates the contribution of each product feature to the decision choice and calculates the unique contribution of each feature to that choice, uncontaminated by its association with other features [20]. Upon testing multiple different options, the optimal choice set was 36 trials with three levels for cost, three levels for external opinions and four levels for accessibility scores. Cost and external opinions both include three levels – high, medium and low. The accessibility levels include no scores, low SMC (sensory-motor-cognitive scores), medium SMC scores, and high SMC scores.

Two case studies determined participants’ preference for medical device purchase and use, based on device attributes. The first case study was for a person with an impairment purchasing a blood pressure monitor. The second case study was for a person trying to schedule a physician’s visit where the type of examination table is a key factor in deciding which physician to choose. The levels for cost were chosen based on real-life estimates of blood pressure monitors and co-pays over insurance coverage for a physician’s visit. The information for external opinions were provided as a star rating of one-star, three-star or five star and was described as “these ratings were provided by other individuals with the same functional abilities as you”. Accessibility scores were provided as no, low, medium or high accessibility. An example of a question related to medical device purchase is shown in Fig. 1.
Evaluation and RESULTS
Multinomial SAS macros and syntax were used to determine the participants’ preferences for devices with differing accessibility scores. Participants were recruited from disability registries and agencies across the country. Ninety-eight individuals with disabilities rated the case study for blood pressure monitors while 63 rated the case study for examination tables. Of the 98 participants, 45 were male and 53 were female. Participants were obtained from ages 18 to over 60, from all socio-economic strata (high to very low), and from 13 impairment groups. The discrete choice model analysis SAS procedure used to analyze the data was PROC PHREG (proportional hazards regression) [21].
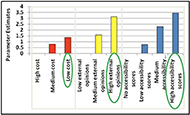
In a discrete choice study, information from each of the choices made by participants is used to obtain a likelihood value for attributes, levels of attributes and their interactions. Results for both devices showed that participants were more likely to prefer devices with the lowest cost, highest external opinions and highest accessibility (p < 0.001). Fig. 2 shows a graphical description of the importance of the levels of the attributes on participants’ decisions for blood pressure monitors.
They also preferred devices where accessibility information was provided versus if it was not (p < 0.001). In the interactions of cost and accessibility and external opinions and accessibility, participants always choose devices with the highest accessibility (p < 0.001), except when the external opinions were low.
DISCUSSION AND CONCLUSION
The overall purpose of this study was to determine the effects of accessibility score information on decisions of individuals with disabilities related to purchase and use of medical devices. Decisions related to the choice of products are important not only for consumers, but also for marketers and policymakers [22]. Research has shown that provision of more information leads to choices being more in line with preferences, and a reduction in uncertainty regarding the nature of product attributes during the choice process [23]. The importance of this information increases for consumers with disabilities who currently do not receive any information related to the accessibility of products they purchase.
Accessibility scores in this study were reported as percentages for individuals with motor, sensory and cognitive impairments. Therefore, participants could identify the category they belonged to and determine the level of accessibility for the device they were choosing. The results obtained from evaluating participants preferences for purchasing both medical devices consistently showed that individuals with disabilities prefer devices with high levels of accessibility compared to devices with lower scores, except in cases when the external opinions about the device were low. These results indicate that accessibility of the device, and consequently the ability to use the device, is extremely important to individuals with disabilities. The study first highlights a need for standards to be developed for accessibility of medical devices. It also has significant labeling implications. Labeling significantly influences product design, advertising, consumer confidence in the product, and consumer education [24]. This study has shown that provision of accessibility information, which may be implemented as a label, significantly effects decisions related to purchase of medical devices for people with disabilities.
The study also has a direct link to the Patient Protection and Affordable Care Act which requires certain types of medical devices including examination tables and chairs, weight scales, x-ray machines and other radiological equipment, and mammography equipment to be accessible for persons with disabilities, with regulations that these be implemented within two years [15]. As accessible medical devices continue to be manufactured, information about accessibility needs to be provided to consumers.
The design of accessible medical devices and provision of consumers with accessibility information has tremendous health care implications. Consumers with disabilities currently report inadequate health care; however, providing them with facilities that they are able to use will increase their use of health care services and maintenance of overall health. There is a need for consumers with disabilities to receive information about accessibility of medical devices that will meet their needs. This issue should be adopted by designers as well as federal agencies to ensure that standards for accessibility of medical devices are developed, met and provided to consumers.
REFERENCES
- United States Census Bureau. American Community Survey - 2018: ACS 1-Year Estimates Data Profiles.
- Lin M, Shaewitz D, Overton C, Smith D-M. A Hidden Market: The Purchasing Power of Working-Age Adults with Disabilities. Washington, DC; 2018.
- Scarborough-Kaufman C. Publicly-Researchable Accessibility Information: Problems, Prospects and Recommendations for Inclusion. Soc Incl. 2019;7(1):164-172. doi:10.17645/si.v7i1.1651
- Cheng E, Myers L, Wolf S, et al. Mobility impairments and use of preventive services in women with multiple sclerosis: observational studies. Br Med J. 2001;323(7319):968-969. doi:10.1136/bmj.323.7319.968
- Coyle CP, Santiago MC. Healthcare utilization among women with physical disabilities. Medscape Gen Med. 2002;4(3):2.
- Mendelson, S. (2007). Role of tax law in the development and use of accessible medical instrumentation. In J. M. Winters & M. F. Story, (Eds.), Medical instrumentation: Accessibility and usability considerations (59-82). Boca Raton, FL: CRC Press.
- Iwarsson, S. & Stahl, A. (2003). Accessibility, usability and universal design: positioning and definition of concepts describing person-environment relationships. Disability and Rehabilitation, 25, 57-66.
- RERC-AMI. (2006). Resources on accessible/universal design principles and performance measures. Retrieved from http://www.rerc-ami.org/ami/projects/d/2/udg/
- DeJong, G. (1997). Primary care for persons with disabilities: an overview of the problem. American Journal of Physical Medicine and Rehabilitation, 76.
- Markwalder, A. (2005). Disability rights advocates. A call to action: A guide for managed care plans serving Californians with disabilities. Oakland, CA: DRA.
- Gans, B. M., Mann, N. R., & Becker, B. E. (1993). Delivery of primary care to the physically challenged. Archives of Physical Medicine and Rehabilitation, 74, s15-s19.
- Ramirez, A., Farmer, G. C., Grant, D., & Papachristou, T. (2005). Disability and preventative cancer screening: Results from the 2001 California health interview survey,” American Journal of Public Health, 95, 2057-2064.
- Story, M. F., Winters, J. M., Premo, B., Kailes, J. I., & Winters, J. M. (2003). Understanding barriers to healthcare caused by inaccessible medical instrumentation. Proceedings of the RESNA Annual Conference.
- Smith, R. O., Barnekow, K., Lemke, M. R., Mendonca, R., Winter, M., Schwanke, T. & Winters, J. M. (2007). Development of the MED-AUDIT (Medical Equipment Device-Accessibility and Universal Design Tool). In J. M. Winters & M. F. Story, (Eds.), Medical instrumentation: Accessibility and usability considerations (283-296). Boca Raton, FL: CRC Press.
- Patient Protection and Affordable Care Act 42 U.S.C. § 18001. Patient Protection and Affordable Care Act, 42 U.S.C. § 18001.; 2010.
- United States Access Board. Standards for Accessible Medical Diagnostic Equipment. Washington, DC; 2010.
- United States Access Board. VA Adopts New Standards for Medical Diagnostic Equipment.
- Ben-Akiva, M. & Francois, B. (1985). Discrete choice analysis: Theory and application to travel demand. Cambridge, Masschusetts: MIT Press.
- U.S. Department of Transportation (1999). Guidebook on methods to estimate non-motorized travel: Supporting documentation. Retrieved from http://www.tfhrc.gov/safety/pedbike/vol2/title.htm
- Kuhfeld, W. F. (2009). Discrete choice. Retrieved from http://support.sas.com/techsup/technote/mr2009f.pdf
- Ang, J. (2006). Discrete Choice Modeling with PROC MDC]. Retrieved from http://analytics.ncsu.edu/sesug/2007/DM08.pdf
- Bettman, J. R., Johnson, E. J. & Payne, J. W. (1991). Consumer decision making. In T. S. Robertson & H. H. Kassarjian, (Eds.). Handbooks of Consumer Theory and Research (50-84). Upper Saddle River, New Jersey: Prentice Hall, Inc.
- Teisl, M. F. & Roe, B. (1998). The economics of labeling: An overview of issues for health and environmental disclosure. Agricultural and resource economics review, 27, 140.
- Caswell, J. A. & Mojduszka, E. M. (1996). Using informational labeling to influence market for quality in food products. American Journal of Agricultural Economics, 78, 1248-1253.