Soonjae Ahn, Inhyuk Moon
Robot and Automation Engineering Major, Dong-Eui University, Korea
Institute of Smart Rehabilitation Engineering and Assistive Technology, Dong-Eui University, Korea
ABSTRACT
Recently, care robots are being developed that incorporate robotics into assistive products that focus on daily care for the physically disabled or elderly with reduced physical function. However, although care robots can reduce the physical burden of human intervention, they can also be dangerous depending on their situational awareness. This study describes a standardization that defines safety requirements for care robots and includes verification methods to test their safety requirements. As an example of the application of this standard, a standard for the safety and performance method of a feeding robot is shown. This standardization study is expected to contribute to the spread of care robots in the future.
INTRODUCTION
The classification and terminology of assistive products are addressed explicitly in the ISO 9999 standard [1]. The standard defines an assistive product as "optimizing a person's functioning and reducing disability." In other words, an assistive product can optimize a person's functioning or reduce disability. With the recent development of robotics, many assistive devices are incorporating robotics to improve their functionality. The terms robot and robotics are well-defined in ISO 8373:2021 [2].
A robot is defined as "programmed actuated mechanism with a degree of autonomy to perform locomotion, manipulation or positioning," and robotic technology is defined as "practical application knowledge commonly used in the design of robots or their control systems, especially to raise their degree of autonomy" [2]. Since IEC/TR 60601-4-1 defines a medical robot as a "robot intended to be used as medical electrical equipment or system," an assistive robot can be defined as a "robot intended to be used as an assistive product" [3]. Similarly, a care robot is a "robot intended to be used for caring persons with disability or elderly persons."
These care robots are intended to support the care, nursing, and physical activities of the care recipient, easing the burden of caregiving and improving the quality of life of the elderly or disabled and their families. The care robots are developed around basic activities such as Feeding, Personal toileting, Hoist, and Posture-changeable robots. However, there are still no clear definitions, products classifications, and safety standards for care robots, so their dissemination has many difficulties.
In this study, a care robot is defined as "a robot to assist activities of daily living for persons with disability or the elderly who have difficulties in daily life". However, although care robots can be physically burdensome by reducing human intervention, they can also be dangerous depending on their ability of situation awareness. Therefore, we propose a standard that defines safety requirements specific to care robots and includes verification methods to test safety based on the safety requirements. As an example of applying this standard, we show a standard of safety requirements and performance tests for feeding robots.
As for standards related to care robots, the general standard for assistive products, ISO 21856, was recently published [4]. It consists of 29 clauses with general requirements in clause 4 and individual requirements in clauses 5 through 26. The typical international standard for medical rehabilitation robots is IEC 80601-2-78 [5]. This standard requires mechanical hazards' safety by robots based on the mechanical hazards described in IEC 60601-1 which is a general standard for electrical medical devices. It is necessary to refer to it in the care robot standard because it considers the characteristics of robots. IEC 60601-1 states that the primary user may be a patient and a clinical professional. Therefore, for medical robots with a degree of autonomy (DOA), the user's situational awareness can be an important safety factor [6]. Since the primary users of care robots are lay persons as called care receivers, or caregivers, the risk by the loss of situational awareness should be considered. To verifiy these risks, usability engineering process is necessary as shown in medical devices [7].
Medical devices, assistive devices, and children's products that have been used for a long time have well-documented safety requirements. However, the field of care robots, which has a short history, still lacks safety standards. This study proposes standards for safety and verification methods suitable for care robots by referring to existing standards.
CARE ROBOT
Definition of care robot
Care or care service is defined as "the act of providing physical or mental assistance to the elderly or disabled who have difficulty maintaining daily activities on their own."
Thus, a care robot can be defined as "a robot to assist activities of daily living for persons with disability or the elderly who have difficulties in daily life".
Types of care robots
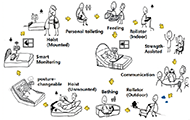
Care robots include feeding robots, hoist robots, personal toileting robots, posture-changeable robots, and rollator robots to reduce the burden on caregivers and improve the quality of life of care recipients [Figure 1] [9].
SAFETY STANDARDS FOR CARE ROBOTS
ISO 8373 [2] defines a robot as "programmed actuated mechanism with a degree of autonomy to perform locomotion, manipulation or positioning." Based on this definition, a care robot can be considered a robot as "an assistive device or drive mechanism for daily living with a degree of autonomy." Based on this definition, the necessary safety requirements and tests were investigated, analyzed, and reflected in the standard.
Structure of care robot standards
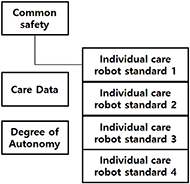
The structure of care robot standards is divided into a common (general) safety standard, individual (particular) safety standards, care data standards, and care robot degrees of autonomy [Figure 2]. The common safety standard for care robots covers general safety requirements that should be applied to care robots. In contrast, the individual care robot standard covers requirements that are difficult to apply in general in consideration of the characteristics of individual care robots and must be considered for each item. The care data standard and the care robot degree of autonomy standard are separate standards that can be referenced for care robots.
General requirements
For risk analysis and management of care robots, the procedures of ISO 14971 or ISO 12100 are applied [10,11]. In addition, for care robots classified as medical devices, IEC 62366-1 is used to identify hazardous situations [7], and for care robots with embedded programs, IEC 62304 is used [12]. In particular, for care robots with degree of autonomy, the loss of the user's situational awareness is considered a hazard [13].
Multifunctional care robots with two or more functions must meet the safety and performance requirements in individual standards according to the primary function proposed by the manufacturer. Functions other than the primary function shall be classified as minor and meet common safety requirements.
If the care robot supports wired and wireless communication (Wi-Fi, Bluetooth, USB, RS-232, LAN, etc.), it must be designed for availability, confidentiality, and integrity within the risk management process.
Safety requirements for care robots

Safety requirements are categorized into electrical safety, mechanical safety, cleaning and disinfection, environmental factors, and hazardous materials.
Electrical safety is required to comply with national safety certification requirements. If there are no national safety certification requirements, at least meet the requirements for leakage current, withstand voltage, and over temperature. In addition, built-in batteries, emergency stop, protectives stop, and electronics compatibility are required [Table 1]. Mechanical safety requirements are provided for the structure, pinching, squeezing, and restraint to ensure that the user of the care robot is not at risk of mechanical harm.
| Item | Requirements |
| Built-in battery |
|
| Emergency stop |
|
| Protection stop |
|
Requirements for hazardous substances are provided for parts of the robot that come into contact with the human body. The allowable values of hazardous substances are based on the Korean certification (KC) standard for children's products [8].
To prevent harm from noise, the standard requires a maximum noise level of no more than 50 dB in sleeping environments, 65 dB indoors, and 85 dB outdoors. In addition, the standard requires classification for water resistance according to IEC 60529 [13].
EXAMPLE OF FEEDING ROBOT
This section introduces the individual requirements of a feeding robot. The shape of a feeding robot is shown in Figure 3. The safety of the feeding robot is further tested for impact energy, static load, and repetitive durability. The performance of the feeding robot is further evaluated through pose accuracy (ISO 9283)[14], continuous use time, and feeding assistance success rate.
CONCLUSION
Recently, various research projects on care robots have been started and are in progress. However, due to the lack of relevant standards, there may be problems with the steps for licensing or certification for future productization. Therefore, we developed a standard for safety requirements for care robots in this study.
The requirements for electrical safety and mechanical safety, which are safety requirements for medical devices, were investigated and applied [6]. The mechanical risk factors defined by robots were additionally reflected by referring to ISO 13482 [17]. In addition, since care robots use robotics and the primary users are non-experts, the risk of situational awareness loss was considered by referring to IEC 80601-2-78, an international standard for rehabilitation robots [5], and cybersecurity was also added. Performance issues will be addressed in individual standards for care robots to be developed in the future.
In a situation where standardization of safety evaluation of care robots is actively needed, it is expected that securing product safety and consumer trust through normal development will be very important for the successful settlement and expansion of the care robot industry. Furthermore, the developed standards can be used for licensing and establishing policies for care robots, and manufacturers will be able to ensure safety in the development stage of care robots.
The addition of robot and robot technology definitions, automatic toileting systems, power assist units for assistive products for walking, and feeding robots to ISO 9999 [1] has paved the way for developing and institutionalizing international standards for care robots. Therefore, it can be promoted as an international standard based on the current standards. In addition, we will also develop standards for individual care robot items so that care robots currently under development can be released to the market with guaranteed safety and performance.
REFERENCES
[1] ISO 9999:2022, Assistive products - Classification and terminology.
[2] ISO 8373:2021, Robots and robotic devices — Vocabulary.
[3] IEC 60601-1-4, Medical electrical equipment — Part 4-1: Guidance and interpretation — Medical electrical equipment and medical electrical systems employing a degree of autonomy
[4] ISO 21856:2022, Assistive products - General requirements and test methods.
[5] IEC 80601-2-78:2019, Medical electrical equipment — Part 2-78: Particular requirements for basic safety and essential performance of medical robots for rehabilitation, assessment, compensation, or alleviation.
[6] IEC 60601-1:2022, Medical electrical equipment - ALL PARTS.
[7] IEC 62366-1:2015 Medical devices — Part 1: Application of usability engineering to medical devices.
[8] Korean Agency for Technology and Standards. Special Act on The Safety of Children's Products Annex 6 (Toys – 21.7.1).
[9] W. K. Song, S. C. Kwon, and G. O. Ahn, "Research on planning to establish a foundation for care services including robot technology," Korea, National Rehabilitation Center, pp. 13, 2018.
[10] ISO 14971:2019, Medical devices — Application of risk management to medical devices.
[11] ISO 12100:2010, the safety of machinery — General principles for design — Risk assessment and risk reduction.
[12] IEC 62304:2006+AMD1:2015 Medical device software - Software life cycle processes.
[13] IEC 60529:1989/AMD2:2013/COR1:2019, Degrees of protection provided by enclosures (IP Code).
[14] ISO 9283 Manipulating industrial robots — Performance criteria and related test methods.
[15] IEC 62133-2 Secondary cells and batteries containing alkaline or other non-acid electrolytes - Safety requirements for portable sealed secondary cells, and for batteries made from them, for use in portable applications - Part 2: Lithium systems
[16] IEC 60417 - 5638, Emergency stop
[17] ISO 13482:2014 Robots and robotic devices — Safety requirements for personal care robots.
ACKNOWLEDGMENT
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HJ20C0040, HI21C0484).